How to convert hydrogen peroxide by volume into hydrogen peroxide percentage.
Hydrogen peroxide concentration can be denoted in many different ways. Two of the most common ways are by percentage and by volume. This can add confusion to an already confusing topic.
So the concentration is the amount of hydrogen peroxide in a solution and hydrogen peroxide when it decomposes releases oxygen , a single atom of oxygen and the rest is water.
Hydrogen peroxide releases oxygen at a specific rate 3.3 volumes per percentage. So a 1% hydrogen peroxide will release 3.3 volumes of oxygen.
So all we have to do is remember this which is probably the harder part take your volume and divide it by 3.3 to get your hydrogen peroxide concentration.
So in the hair styling industry and Europe they go by volume a lot and so a 10 volume hydrogen peroxide divide that by 3.3 and we get a 3.03% concentration of hydrogen peroxide.
A 20 volume hydrogen peroxide would equal a 6.06% concentration.
A 60 volume hydrogen peroxide would equal a 18.18% concentration hydrogen peroxide.
Using 35% Hydrogen Peroxide As Chlorine Alternative In Hot Tub
35% Spa Science Hydrogen Peroxide is a great chlorine alternative. 4 oz per 100 gallons of hot tub water every 3-4 days.
A 100 ppm hydrogen peroxide level must be maintained at all times. You may exceed 100ppm safely. Our 0– 400ppm hydrogen peroxide test strips are an easy way to ensure minimum levels are maintained.
When switching and using 35% Hydrogen Peroxide as a chlorine alternative we recommend starting with fresh water.
Also bi-weekly filter flushes help reduce early decomposition, resulting in less hydrogen peroxide needed to maintain optimal levels.
Our 35% Spa Science is 35% Food Grade Hydrogen Peroxide labelled for intended use.
Benefits of using 35% hydrogen peroxide as a chlorine alternative.
Chlorine is a common disinfectant used in water treatment and swimming pools. However, chlorine can produce harmful byproducts, such as trihalomethanes (THMs), which are linked to cancer and other health problems.
35% hydrogen peroxide is a safe and effective when diluted properly. It is a powerful oxidizer when contacting organic material. Organic material includes wood, soil, agea, bacteria, viruses, and other pathogens.
Hydrogen peroxide breaks down into harmless water and oxygen, so it does not produce any harmful byproducts.
Here are some of the benefits of using 35% hydrogen peroxide as a chlorine alternative:
- Safer for your health: Hydrogen peroxide is a non-toxic at low concentrations and a biodegradable substance. 35% Hydrogen peroxide can be dangerous and must be handled and stored appropriately. DO NOT DRINK 35% hydrogen peroxide. Federal water treatment levels below 1100ppm are considered GRAS generally regarded as safe. 1100ppm is 0.1100%. Most of us have experience with 3% hydrogen peroxide and the bubbling it causes when poured over a scrape or cut. 35% is 10 times more concentrated! 35% hydrogen peroxide is a very strong oxide and corrosive and must be handle with care. When diluted properly It is safe for humans, animals, and plants. Chlorine, on the other hand, can irritate the skin, eyes, and respiratory system. It can also be harmful to aquatic life.
- More effective oxidizer. Hydrogen peroxide is more effective at oxidizing unwaa wider range of pathogens than chlorine. It is also effective at killing pathogens that are resistant to chlorine.
- More environmentally friendly: Hydrogen peroxide does not produce any harmful byproducts, so it is safer for the environment than chlorine. Chlorine can produce THMs and other harmful byproducts that can pollute waterways and harm aquatic life.
- Less corrosive: Hydrogen peroxide is less corrosive than chlorine, so it is safer for pipes and other equipment. Chlorine can corrode pipes and equipment, which can lead to leaks and other problems.
How to use 35% hydrogen peroxide as a chlorine alternative
35% hydrogen peroxide can be used as a chlorine alternative in a variety of applications, including:
- Water treatment: Hydrogen peroxide can be used to disinfect drinking water, wastewater, and swimming pool water.
- Food processing: Hydrogen peroxide can be used to disinfect food surfaces and equipment. It can also be used to kill pathogens on fruits and vegetables.
- Healthcare: Hydrogen peroxide can be used to disinfect medical instruments and surfaces. It can also be used to clean wounds.
Conclusion
35% hydrogen peroxide is a safe and effective alternative to chlorine. It is more effective at killing pathogens, more environmentally friendly, and less corrosive than chlorine. 35% hydrogen peroxide can be used in a variety of applications, including water treatment, food processing, and healthcare.
Important safety information
35% hydrogen peroxide is a concentrated solution and can be irritating to the skin, eyes, and respiratory system. It is important to wear safety glasses and gloves when working with 35% hydrogen peroxide. If you get 35% hydrogen peroxide on your skin, wash it off immediately with soap and water. If you get 35% hydrogen peroxide in your eyes, flush them immediately with water for 15 minutes. If you inhale 35% hydrogen peroxide, get fresh air immediately. If you experience any discomfort after being exposed to 35% hydrogen peroxide, seek medical attention.
Hydrogen Peroxide Material Compatibility
35% Hydrogen Peroxide undiluted is an oxidizer and corrosive. Most hot tubs use newer material for seals and gaskets which are compatible with hydrogen peroxide. Although the concentration level is very low after adding 35% Hydrogen Peroxide to a hot tub knowing what materials are best is always good.
Natural rubber is not compatible with hydrogen peroxide. The rubber will break down fairly quickly and the reason “eye droppers” should not be used to dispense 35% Hydrogen Peroxide.
For Hydrogen Peroxide 30% +. A “C” rating may work for some industrial application however we do not recommend using materials with a “C” or “D” as to avoid possible unwanted chemical residue from material degradation.
| Material | Compatibility |
|---|---|
| ABS plastic | N/A |
| Acetal (Delrin®) | D – Poor |
| Aluminum | A – Excellent |
| Brass | N/A |
| Bronze | B – Good |
| Buna N (Nitrile) | D – Poor |
| Carbon graphite | C -Not Rec. |
| Carbon Steel | D – Poor |
| Carpenter 20 | B – Good |
| Cast iron | B – Good |
| Ceramic Al203 | N/A |
| Ceramic magnet | A – Excellent |
| ChemRaz (FFKM) | B – Good |
| Copper | D – Poor |
| CPVC | A – Excellent |
| EPDM | B – Good |
| Epoxy | B – Good |
| Fluorocarbon (FKM) | A – Excellent |
| Hastelloy-C® | A – Excellent |
| HDPE | A -Excellent |
| Hypalon® | D – Poor |
| Kalrez | A – Excellent |
| Kel-F® | B – Good |
| LDPE | C – Not Rec. |
| Natural rubber | D – Poor |
| Neoprene | D – Poor |
| NORYL® | A – Excellent |
| Nylon | D – Poor |
| Polycarbonate | A – Excellent |
| Polyetherether Ketone (PEEK) | A – Excellent |
| Polypropylene | B – Good |
| Polyurethane | B – Good |
| PPS (Ryton®) | A – Excellent |
| PTFE | A – Excellent |
| PVC | A1 – Excellent |
| PVDF (Kynar®) | A – Excellent |
| Silicone | B – Good |
| stainless steel – 304 | B – Good |
| stainless steel – 316 | B – Good |
| Titanium | B – Good |
| Tygon® | B – Good |
| Viton® | A – Excellent |
Ratings – Chemical Effect
A – Excellent
B – Good: Minor Effect, slight corrosion, or discoloration.
C – Moderate Effect, not recommended .
D – Severe Effect: Not recommended for any use.
There are many types of elastomer material the list above are some of the more common.
Oxygen In The Muscles
How Low Oxygen Contributes To Fatigue
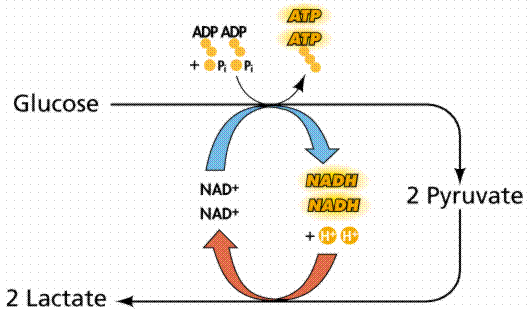
Oxygen is used in so many process in the body. Every cell in the body contains mitochondria that uses oxygen to convert glucose (sugar) into energy. This process is called cell respiration.
When oxygen falls below optimal levels such as during exercise or physical exertion normal aerobic cell respiration will switch to a less efficient method of converting glucose to energy called lactic acid fermentation. This is a type of anaerobic cell respiration which does not require oxygen. Continue reading “Oxygen In The Muscles”
Chlorine Alternative For Your Hot Tub
A chlorine alternative or bromine alternative makes sense for a healthier life. Research has begun to show adverse health risk linked to the use of chlorine as a water sanitizer. Inhibiting the endocrine system is especially harmful for women.
The chlorine molecule mimics a key hormone with receptors in the thyroid gland causing an over or under releasing of hormones. Over produced hormones combined with under produced hormones in this area research has drawn strong relationship to certain types of cancers.
The endocrine systems is made up of all of the bodies hormones. These tiny “chemical messengers” get released into the blood stream and act on other organs through-out the body.
The endocrine system regulates many processes including reproductive, temperature, mood, metabolism and blood sugar levels. Just because we’ve used chlorine as a hot tub chemical in the past doesn’t mean we should continue.
Chlorine Alternative
35% Spa Science Hydrogen Peroxide can be used as not only an alternative to chlorine or bromine but as the primary choice for hot tubs and spas! Hydrogen Peroxide has been known and accepted as a sanitizer for many years.
35% Spa Science Hydrogen Peroxide is 35% Food Grade Hydrogen Peroxide intended to be used as a water additive in maintaining hot tub water for cleaning purposes..
Soaking in clean oxygen rich water, that 35% Spa Science can offer, is a great way to enjoy your spa. When diluted properly 35% Spa Science is a non-toxic hot tub chemical and at low concentrations is actually formed naturally in nature and our bodies. Hydrogen Peroxide is the bodies first defense in fighting off disease.
Although this product is not intended to be used typically to cure, diagnose treat or prevent disease it may indirectly achieve the aforementioned by eliminating the use of chlorine and bromine!
Chlorine And Disinfection By Products
Chlorine when used to disinfect water can react with certain organic matter and produce hundreds of chemical by-products. These chemical by-products are know as DBP’s. Two major classes of DBP’s, trihalomethanes (THMs) and haloacetic acids (HAA), make up the bulk of these by-products.
Research has Continue reading “Chlorine And Disinfection By Products”