How human biology creates hydrogen peroxide is fascinating. This article is a general overview to the best of our understanding.
Our products are NOT intended to diagnose, treat, cure or prevent disease and this must not be interpreted as medical advise.
Detecting Hydrogen Peroxide In Blood And Plasma
The levels of hydrogen peroxide (H2O2) in human blood is of great relevance as it has emerged as an important signalling molecule in a variety of disease states. Scientist have been working on reliable ways to measure hydrogen peroxide levels in the blood. Although challenging advancements in a laser-induced fluorescence device has demonstrated rapid measurement of H2O2 in plasma in the concentration range of 0–49 µM, and detection time of 15 min; the device is amenable to the real-time measurement of H2O2 in a patient’s blood. (1)
H2O2 concentration in the blood of healthy individuals is found to be in the range of 0.8–6 µM. Hydrogen peroxide (H2O2) is one of the important ROS which is produced due to the incomplete reduction of oxygen in the metabolism process and most cells in the human body generate H2O2 from superoxide. H2O2 is uncharged and stable in aqueous solution and its uncharged nature helps it to diffuse across the cell membrane, enabling cellular signalling away from the site of production.
illness comes in many forms disease vs a cold or flu, H2O2 has been measured in the urine of patients who are showing signs of a cold as high as 100ppm. We look forward to conducting our research if or when that time comes using our 0-400ppm H2O2 test strips. H2O2 has a longer lifespan than other reactive oxidizing agent which allows a greater diffusion level of up to a few millimeters. H2O2 which is diffused out of a cell triggers cell migration, immunity generation, and cellular communication. 2
Blood cells including red blood cells produce H2O2 from multiple sources but the level of intracellular H2O2 is maintained as 10 nM or less due to the catalase and peroxidases1. The plasma H2O2 is mainly contributed by NOXs (nicotinamide adenine dinucleotide phosphate oxidase) on the surface of phagocytes and endothelial cells and xanthine oxidase bound to endothelial cells with a small contribution from autoxidation of small molecules. 3
Respiratory Burst
The human body uses a biologic process called the respiratory burst to generate hydrogen peroxide in the immune system. The respiratory burst is a rapid increase in the production of reactive oxygen species (ROS), including hydrogen peroxide, by phagocytic cells such as neutrophils and macrophages.
Triggering Phagocytic Immune Cells
The respiratory burst is triggered by the activation of phagocytic cells by pathogens or other foreign invaders. Once activated, the phagocytic cell engulfs the pathogen in a phagosome. The phagosome then fuses with a lysosome, which releases enzymes that kill the pathogen. The respiratory burst also produces ROS, which help to kill the pathogen and prevent it from escaping from the phagosome.[su_tooltip text=”In cell biology, a phagosome is a vesicle formed around a particle engulfed by a phagocyte via phagocytosis. Examples of phagocytes include macrophages, neutrophils, and dendritic cells”]Hover me to open tooltip[/su_tooltip]
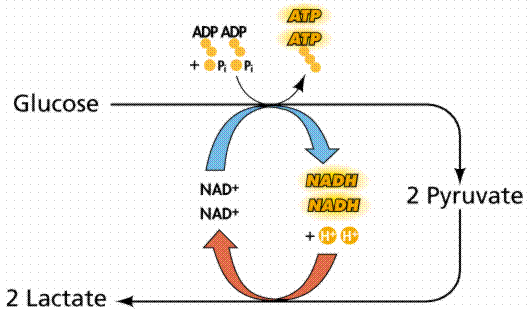
The respiratory burst is mediated by a group of enzymes called NADPH oxidases (NOXs). NOXs use NADPH to produce superoxide, which is then converted to hydrogen peroxide by superoxide dismutase (SOD). Hydrogen peroxide can then be used by other enzymes to produce other ROS, such as hydroxyl radicals.
Hydrogen peroxide is a powerful antimicrobial oxidizing agent. It can oxidize bacteria, viruses, and other pathogens by damaging their DNA and proteins. Hydrogen peroxide also plays a role in cell signaling and inflammation.
Here is a simplified summary of the respiratory burst process:
- Phagocytic cell is activated by a pathogen or other foreign invader.
- Phagocytic cell engulfs the pathogen in a phagosome.
- Phagosome fuses with a lysosome, which releases enzymes that kill the pathogen.
- NADPH oxidases produce superoxide, which is then converted to hydrogen peroxide by SOD.
- Hydrogen peroxide can then be used by other enzymes to produce other ROS.
- Hydrogen peroxide and other ROS kill the pathogen and prevent it from escaping from the phagosome.
The respiratory burst is an important part of the human body’s immune system response. It helps to protect us from infection by destroying harmful pathogens.